
Founded in July 1995, Jingjia Medical is a technology-driven manufacturer specializing in medical-grade sodium hyaluronate. With nearly three decades of experience, we focus on the development and manufacturing of CE-certified sodium hyaluronate gels, including aesthetic dermal fillers and ophthalmic viscoelastic devices.
Our brand HAFILLER is recognized for its quality and performance in both medical and aesthetic applications.
Our products are supplied across China and exported to over 40 countries and regions worldwide, serving partners in Europe, South America, the Middle East, Southeast Asia, and CIS markets.
Guided by continuous innovation and strict regulatory compliance, Jingjia Medical is committed to becoming a trusted global supplier of injection-grade and medical-grade sodium hyaluronate for medical and aesthetic applications, and an oem services provider.
Zhejiang Jingjia Medical Technology Co., Ltd. was founded with a focus on the research, development, manufacturing, and commercialization of biomedical materials.
Jingjia Medical began early development and regulatory approval of hyaluronic acid–based products for ophthalmic surgery, intra-articular injection, and anti-adhesion applications, establishing a strong foundation in medical-grade biomaterials.
Jingjia Medical achieved ISO 9001 and ISO 13485 quality management system certifications, ensuring compliance with international medical device quality standards.
HAFILLIER medical-grade sodium hyaluronate gel received CE certification, enabling entry into the European Union market and marking a major milestone in the company’s global expansion.
Jingjia Medical participated in the development of industry standards for cross-linked hyaluronic acid gels used in aesthetic and reconstructive medical procedures.
Jingjia Medical successfully passed the Medical Device Single Audit Program (MDSAP), meeting regulatory requirements across multiple international markets.
Jingjia Medical further strengthened its regulatory portfolio by obtaining additional Class III medical device registrations and was recognized for its innovation capabilities in medical biomaterials.
The intelligent manufacturing base for medical-grade sodium hyaluronate products was officially launched, enhancing production capacity, automation, and quality consistency.
Jingjia Medical specializes in medical-grade sodium hyaluronate gels for applications including surgical anti-adhesion, ophthalmic viscoelastic devices, joint lubrication, and minimally invasive aesthetic fillers.
Since 1995, we have focused on R&D of sodium hyaluronate technology, including the production of injection-grade raw materials. Our products are known for stable quality, safety, and consistent performance in regulated medical applications, making Jingjia Medical a long-established manufacturer in China’s medical sodium hyaluronate sector.
In November 2014, we established the R&D Center for Cross-linking Sodium Hyaluronate, investing in advanced equipment and specialized talents todrive innovation. Our R&D Center for Cross-linking Sodium Hyaluronate has received recognition for its technological innovation:
This recognition reinforces the company’s capabilities in product development and technological innovation.
Jingjia Medical has invested heavily in establishing a complete and advanced production chain, ensuring that every batch of sodium hyaluronate meets strict international standards.

Sterile production environment with particle count strictly controlled below 100 per cubic foot, ensuring maximum product purity.

High-precision filling machines from OPTIMS (Germany) and terminal sterilizers from FEDEGARI (Italy) enhance reliability and consistency.
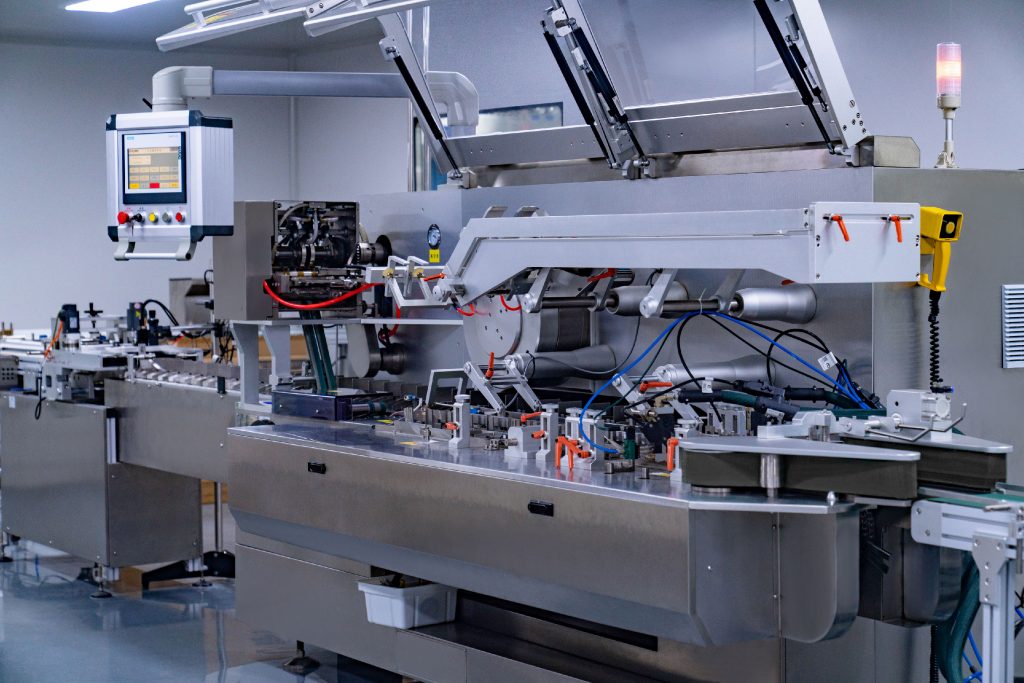
High-efficiency automated packaging ensures consistent quality and an annual output of over 10 million units for domestic and international markets.

Through an advanced purification process, our sodium hyaluronate achieves ≥95% purity and meets the highest industry standards.
Jingjia Medical operates modern, large-scale production bases dedicated to sodium hyaluronate manufacturing:
Covers 87 acres with an annual output of 500 kg of medical-grade sodium hyaluronate raw materials.
Covers 23.85 acres with an annual capacity of 10 million syringes of injection-grade hyaluronic acid products.
These two production bases enable a stable and scalable supply for global medical and aesthetic medicine partners.
Looking for bulk dermal fillers or OEM services? Contact us for flexible cooperation plans.

Zhejiang Jingjia Medical Technology Co., Ltd. was established in July 1995. It is a national high-tech enterprise integrating R&D, manufacturing and sales of biomedical materials.
Product center
Contact Us
Building 3, No. 1288-86, Jingwu Road, Linjiang Street, Qiantang District, Hangzhou, Zhejiang, China
Copyright © 2023 Zhejiang Jingjia Medical Technology Co., LTD Policy | ICP